The Mulder’s chart: Going beyond NPK
By Dan Austin
It is an unfortunate reality that to help us remember things easily, sometimes topics are over-simplified. The five food groups and the proportions we were told to eat growing up for example. Teachings that were simple but have since been adjusted, and found to be far more complex or even downright wrong. The same occurs in horticulture.
When being introduced to plant nutrients, we first cover the big three – nitrogen, phosphorus, and potassium (setting aside hydrogen, oxygen and carbon which are supplied by the environment). These are the nutrients represented in the NPK ratios displayed on any good fertiliser products, as they are required in larger amounts than other nutrients.
My lecturers as a student, bloggers across the web, and many texts, love to highlight that nitrogen is used for leafy green growth and stems, phosphorus is used in root growth and potassium is used for flowers and fruit. The discussion ties up the plant parts nicely, but did I get that wrong? Was it phosphorus that is used for flower production? A quick internet image search will give you a selection of diagrams that will contradict each other. How can such a simple example of nutrient importance be so confusing? The answer is that the diagrams oversimplify nutrient function enormously.
Some diagrams are based on the elemental concentration found in tissue biomass – in this case phosphorous is found in high amounts in roots and flowers. However, other diagrams are based on the function the element performs for the establishment of the organs, in this case potassium (while not physically accumulating in flowers to the level of phosphorus) plays a key role in the developmental health of flowers and fruit. So, the answer is that both nutrients are important for healthy flowers and fruit set. However, even this is an oversimplification. The functions of nitrogen, potassium and phosphorus go far beyond leaves, roots and flowers, and at the same time every essential plant nutrient known, is required for a healthy yield of flowers and fruit not just potassium or phosphorus.
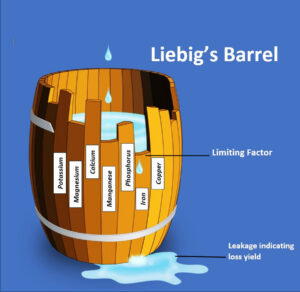
It is a concept that was first conceived in agricultural science more than one hundred and sixty years ago and notably popularised by Justus Von Liebig through ‘Liebig’s Law of the Minimum’. Liebig’s Law states that plant growth is not dictated by the total resources available to a plant, but by the scarcest resource (the plants’ limiting factor). In essence, it suggests that regardless of how much potassium, phosphorus or any other essential element might be available to a plant, its health and yield (including flowers) will be restricted by any element that is lacking, even those needed in only minute amounts. The idea is often illustrated through Liebig’s barrel diagram which illustrates a barrel’s contents flowing out of the lowest point of a wooden plank barrel, each plank a different height and representing a different nutrient, alongside environmental factors like light and water.
So, what if you ensure you have adequate levels of all essential plant nutrients – can you then get a benefit from increasing levels of certain nutrients?
While the answer is sometimes yes, there are many reasons to discourage this way of thinking. Environmentally, excessive fertilising can cause everything from soil salinity and eutrophic waterways to affecting soil microbial and human health. Financially, the cost often outweighs the benefit and very few elements can be added alone. They are more commonly bound as compounds, meaning you are adding (and paying for) more than just the desired element.
Whatever nutrients you add, there is also potential to overdo your application. Fertiliser burn results from the soil solution becoming more saline than the solution within plant roots, causing water to be drawn out of roots through osmosis. Even without reaching toxic levels of salinity, you can cause more harm than good to your plants by adding high levels of specific elements. Nitrogen, for example can lead to large soft foliage at the expense of flowers and fruit, while being highly appealing to piercing and sucking insects. Keeping nitrogen levels in check, maintains hard to digest forms of sugars within the leaves of many plants, detracting insects, and many pest infestations can often be attributed back to earlier, overzealous, fertiliser applications.
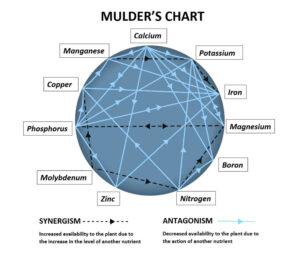
Complicating things further, the over-application of some essential elements can lead to deficiencies of others. This phenomenon is best illustrated by the Mulder’s Chart. Seen in various forms and in varying complexity, the chart is designed to illustrate that in over-supply, many nutrients antagonise the uptake of one another. Elements that act as antagonists can do so in a couple ways. For example, calcium in excess can simply out-compete other elements such as potassium and magnesium for uptake sites on plant roots, or it can change soil chemistry by elevating pH to the point iron and boron become unavailable. Other elements will assist in the uptake of one another, so the diagram is well worth becoming familiar with.
While I started this article by questioning simplistic diagrams, I’ve now offered two more, which, no doubt, the agricultural scientists and organic chemists reading this will regard as over-simplistic too, but hopefully they have you thinking beyond the NPK decal on your fertilisers. While understanding the meaning of the NPK ratios is essential when selecting fertilisers, to get the best out of your plants, all elements need to be made available in the right proportions at the right time. Don’t forget pH and microbial activity can also influence nutrient uptake, so there is a lot at play.
Getting regular elemental soil tests, including pH, are essential for any large-scale production operation and can be just as beneficial for any horticulturist. Maintaining soil health through responsible fertiliser use and fostering the wellbeing of soil biota is horticultural best practice and we should all be doing our part in achieving it.
Dan Austin
IPPS Member, Author
Lecturer, TAFE South Australia
M: 049 122 8591
W: ipps.org
