Feeding plants to fuel growth
By John Fitzsimmons
Centuries of growing experience and science have gone into today’s knowledge of plant nutrition, recorded in innumerable texts, digital records and oral histories. While science is science, the foregoing has also resulted in many interpretations and opinions. This is not the time or channel for a comprehensive review but we can consider a few basic ideas and definitions.
Most living things, including plants, need certain inputs to grow, reproduce, survive and thrive. This includes chemical/nutritional inputs as well as physical inputs such as light, preferred temperature ranges and pressure differentials, and humidity. Plant nutrition has been described as “a difficult subject to understand completely,” in part due to the many differences between species and individual plants.
In addition to light for photosynthesis, and the compounds carbon dioxide and water, there are a number of chemical elements considered ‘essential’ for plant growth. That number has been put at as few as 11 and as many as 20, the latter being most commonly quoted today. For an element to be regarded as essential the following criteria have been listed:
- a plant cannot complete its life cycle without it
- no other element can perform the function of the element
- the element is directly involved in plant nutrition
Essential nutrients are usually categorised as Macronutrients or Micronutrients, the former utilised in larger quantities and the latter in minute quantities (e.g., 0.1 to 200ppm).
| Macronutrients | Micronutrients |
| Carbon (C) | Iron (Fe |
| Hydrogen (H) | Manganese (Mn) |
| Oxygen (O) | Boron (B) |
| Nitrogen (N) | Molybdenum (Mo) |
| Phosphorus (P) | Copper (Cu) |
| Potassium (K) | Zinc (Zn) |
| Calcium (Ca) | Chlorine (Cl) |
| Magnesium (Mg) | Nickel (Ni) |
| Sulfur (S) | Cobalt (Co) |
| Sodium (Na) | |
| Silicon (Si) |
Hydrogen, oxygen, nitrogen and carbon are said to often constitute more than 95% of a plant’s biomass (dry matter basis). The plant’s basic nutrients, carbon, hydrogen and oxygen, are sourced from air and water, the balance mostly from the root zone.
Carbon is needed to form carbohydrates, proteins, starches and cellulose, and many other compounds. The dry weight of a cell is often put at up to 50% carbon.
The next most abundant element in plant cells, nitrogen, forms part of proteins and nucleic acids, and is also used to synthesise some vitamins. It is an essential component of chlorophyll and is often a limiting factor in plant growth.
Hydrogen is used to build sugars. With oxygen it forms water and is part of many organic compounds.
Oxygen is necessary for cellular respiration and plants use oxygen to store energy in the form of ATP (adenosine triphosphate, the principal molecule for storing and transferring energy in cells).
Phosphorus is needed to synthesise nucleic acids and phospholipids. As part of ATP, it enables food energy to be converted into chemical energy. Light energy is converted into chemical energy during photophosphorylation in photosynthesis, and into chemical energy to be extracted during respiration.
Sulfur is part of certain amino acids and plays a role in photosynthesis as part of the electron transport chain (associated with the conversion of light energy into ATP).
Potassium plays an important role in regulating stomatal opening and closing, allowing gas exchange and helping the plant maintain a healthy water balance. It is notably associated with leaves and growing points, and is highly soluble and mobile within the plant.
Calcium helps regulate nutrient transport and supports many enzyme functions. It is mainly found in leaf tissue and is a major constituent of cell walls. Magnesium is important for photosynthesis.
The roots, especially the root hairs, are the principal structure for nutrient uptake. This can also be affected by the structure and architecture of the root/s. Other elements can be taken in from the air through the leaves.
Nutrient uptake from the soil is achieved via cation exchange, where hydrogen ions (+) are transferred into the soil where they displace cations (-) attached to negatively charged soil particles, making the cations available for uptake by the plant via the roots. The stomata in the leaves open to take in carbon dioxide and expel oxygen. The carbon dioxide is used as the carbon source in photosynthesis.
Nutrient ions are then transported to the conducting tissues, xylem and phloem. Xylem moves water and mineral ions in the plant, phloem is responsible for organic molecule transportation.
Water potential, pressure differential, plays a key role in a plant’s nutrient uptake. Lower pressure in the plant than that in the rootzone (soil) will see nutrients moving from the region of higher pressure and nutrient concentration to that of lower pressure and concentration in the plant.
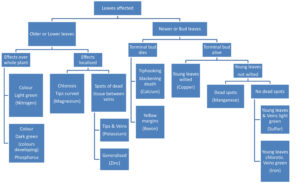
Plants move nutrients to where they are most needed. If the plant is trying to supply more nutrients to younger leaves than older ones, deficiency symptoms will first appear on the older leaves. However because not all nutrients are equally mobile, when less mobile nutrients are deficient the younger leaves suffer first because the nutrient supply tends to be held back in the older leaves.
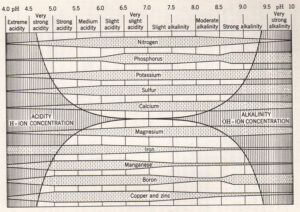
Obviously the original, or basic, nutrient content of the soil will be a major factor determining which plants grow best in that soil, but while nutrient presence might be one thing, nutrient availability (to the plant) can be quite another. Soil pH has a major effect on nutrient availability. Sandy soils have a lower ionic capacity to retain nutrients. Plants also have evolved various relationships with soil microbes, bacteria, fungi and invertebrates which can also affect nutrient uptake, positively or negatively.
At this point it is appropriate to refer to “Liebig’s law of the minimum” (named for Justus von Liebig), which states that growth is dictated not by total resources available, but by the scarcest resource (or limiting factor). It implies that only by increasing the amount of the limiting nutrient, can the growth of a plant or crop be improved. However, it has also been argued that it ignores “the great flexibility of plants to acclimate morphologically and physiologically to changing environmental conditions”, and that, as the plant/crop develops and the environment changes, ‘resource allocation’, plant development and physiology can be dynamic and continually adjusting through the plant’s life cycle. It also implies that under many conditions, a number of resources may be simultaneously limiting crop growth.
This last point should be remembered when setting out to diagnose a plants’ nutritional status, as a number of causes and effects may be overlapping or interacting.
Today there are a growing number of nutrient sources and soil treatments which can complicate plant nutrition decision making. For example, biochar is a charcoal-like substance that’s made by burning organic material from agricultural and forestry wastes in an oxygen-limited environment. Many benefits are claimed for biochar, its porous nature claimed as effective at retaining both water and water-soluble nutrients and providing an attractive habitat for beneficial soil microorganisms. It is promoted as a highly effective vehicle for carbon sequestration. However, depending on use, it can slightly elevate soil pH.
Humic acids are said to have an ability to bind insoluble metal ions, oxides and hydroxides, and to release them slowly and continually to plants when required. Not usually touted as a fertiliser, it is described as “acting as a conditioner for the soil and as a bio-catalyst and bio-stimulant for the plant”. Humic acids’ properties are claimed to produce physical, chemical and biological effects. I have heard of them being used in compost teas.
While generic ideas, these inputs are often available as proprietary products in a variety of forms and formulations, much like the familiar seaweed or kelp-based products combined with added fish extract or, indeed, liquid humates.
Elsewhere in this issue of Hort Journal you will find a mention of TerraCottem®, another soil conditioner with nutrient and moisture retention features.
It becomes a matter of determining your soil’s current status, identifying if there is potential benefit for your plants, and trialling some promising options, with homework and your eyes open.
A hydroponic note
Much of the greenlife industry is rooted (no pun intended) in soil culture. And todays’ market often refers to ‘organic’ growing. However, there are also sectors of the industry that use soilless techniques, and to many people the words ‘organic’ and ‘hydroponic’ cannot live together.
Hydroponic systems allow plants to take up water and nutrients at significantly increased rates compared to soil. This can speed up growth and yield but it also requires nutrients to be constantly available at optimum concentrations, achieved by using inorganic nutrients that the plant can immediately utilise.
Organic growing instead relies on the presence of microbial life to break down complex organic molecules into simpler forms for plant uptake, a longer process, so the nutrients plants need to support such accelerated growth might not be present in sufficient quantities. The result might be uneven or inconsistent growth as the plant accesses some nutrients and not others.
So, if inorganic nutrients are not used, a rich microbial population is needed to ensure the biological reactions necessary to feed the plants at a fast enough rate.
In all cases, growers are striving for healthy well-performing* (*depending on your ultimate product) plants. There are pros and cons for both (easily searched these days) and there are significant differences in how plant nutrition is approached. Ultimately, the plant still needs its essential nutrients and it still accesses those nutrients as it needs, and as it can, under either philosophy.
Note: Sulphur vs Sulfur. The International Union of Pure and Applied Chemistry (IUPAC) deals with questions of nomenclature of chemical elements and compounds. IUPAC decided that element 16 should be spelled as ‘sulfur’ late in the 1900s.
