Humates, chelates and other ‘secret solutions’
By John Fitzsimmons
Most people in horticulture are familiar with the big-ticket plant nutrients, readily focusing on the familiar NPK summaries. However, shelf labels, catalogues, and online searches often include other nutritional references that may be less familiar and/or less understood.
Whether planted in pots, or under cover or outdoors in a broader soil mass, our plants need some nutrition to sustain them in a suitable physical root environment. Some initial ‘natural’ nutrition is usually available in soils and potting mixes, often extended or supplemented by the artificial addition of nutrient sources (e.g. granular fertilisers, slow or delayed release granules etc.) or ameliorants to improve the physical, chemical or biological environment in the root zone (e.g. manures or inoculations).

The adage ‘feed the soil, not just the plant’ relates to this perspective. Especially in soil mass plantings, it is vital to ‘feed’ the broader mass of soil organisms, including microbes, fungi and invertebrates; ultimately, they play a major role in the supply and availability of nutrients to plant roots. Once, soil tests took an essentially empirical inorganic chemistry (industrial?) approach to soil evaluation. Now, for the ‘third leg of the stool’ – the soil’s microbiological status, is usually given more appropriate recognition.
Because of the long-accumulated history of plant nutrition research and contemplation, many articles like this one end up reading or sounding similar. That should not stop anyone trying to better understand their greenlife by reading (and listening) widely, or experiment with the many options and opportunities that will present themselves. While many subterranean processes in the plant’s domain are broadly simple, the chemical interactions can also be amazingly complex, especially in the face of variables like soil pH, temperature, the primary origin of soil/media materials, and microbiology.
Nevertheless, let’s first consider that ‘plants can’t chew their food so they have to drink it’. Go back to basic science and see that plants primarily take in mineral nutrients as ions, mostly through the roots from the soil-water solution. Remember also that about 95% of the plant’s needs are for carbon, hydrogen and oxygen. These are followed by the ‘macronutrients’ including the familiar NPK (nitrogen, phosphorus, potassium) and calcium, sulfur* and magnesium. Then follow the ‘micronutrients’ chlorine, iron, boron, manganese, zinc, copper, molybdenum and nickel. Other nutrients (e.g. silicon) may be beneficial to plant growth but are not essential.
For most plants, with some exceptions, the root system is the main portal for nutrient entry. Some nutrients, mainly micronutrients, can be taken in through the foliage but this is usually secondary to the root system and can vary with the species or category of plant. Micronutrient deficiencies, especially in green leafy crops, are often countered with applications of foliar nutrients. However, foliar nutrition is really deserving of its own article.
So, we are left looking at uptake of nutrients as ions from the root zone.
About chelates
Chelates are organic** chemical structures or complexes – ‘ring systems’ – that have metal ions (+) bonded to chelating agents/molecules at multiple attachment sites. The overall molecular structure is in the form of a ‘claw’ likened to crab claws; the word comes from the Greek word chēlē for claw.
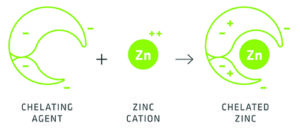
Chelating agents can be naturally occurring or man-made.
Logically, these agents readily accept positively (+) charged ions which include the plant nutrients iron (Fe++), calcium (Ca++), copper (Cu++) and others to form chelates, usually chemically stable and water soluble. The plant’s nutrient entry points on the roots (and leaves) are usually negatively (-) charged which restricts the entry of the (+) charged nutrients. However, in chelated form, presenting a neutral or weakly positive charge, they are more readily admitted to the plant. Hence the (+) charged nutrients are more readily available to the plant. This is why chelated nutrients are often advocated as offering improved availability to plants.
As one saying goes: ‘make sure your plants can eat what you actually feed them’.
And about humates

The bulk of plant nutrients in natural soils originate from weathering and breakdown of the underlying rock. However, a smaller but vital source of nutrients comes from the cyclic waste and breakdown of soil microbes, fungi and invertebrate organisms.
A big part of what they produce are referred to as the humates – humic acid. Its organic chemical composition varies greatly depending on its sources. Humic acid is a chelating agent that is not especially soluble in water but more readily soluble at higher pH (alkaline conditions).
A related product of this activity, a component of the broader humates, is fulvic acid; also a chemically variable chelating agent (not chemically ‘pure’) but usually readily soluble in water at most pH values, lighter in molecular weight than humic acid, and with higher oxygen content. Fulvic acid is generally regarded as more readily absorbed by plants, both in the root zone and via foliar applications.
Both humic acid and fulvic acid are chelating agents and therefore can be significant carriers of (+) plant nutrients and improvers of improved nutrient availability.
Being derived primarily from organic processes this is the origin of the concept of ‘feeding the soil not the plant’. By supporting the production of humates you can improve the supply availability of nutrients to the plant.
Broadly, some of the benefits claimed for humates and chelates include:
- improved nutrient uptake
- prevention and/or remedy of nutrient deficiencies
- improved nutrient availability in situations of inappropriate pH or other soil conditions
- use both in the root zone and through foliar applications
- targeted nutrition via applications and formulations for specific nutrients at appropriate periods of plant growth cycles
- broad compatibility with many NPK fertilisers and pesticides
But as said above, the chemistry of chelating agents, including humates, is diverse and can be naturally occurring or artificially made. There is also a dearth of ‘standardised’ tests and measures of chelates and their effective value. So, it is difficult to say ‘check for this active’ or ‘look for this composition or that value’. This can leave the way clear for poorly supported claims and confusing marketing. The best response is doing your homework well for your specific plant species and situation, and some well-considered comparative trial plots.
*Sulfur is derived from the Latin word sulpur, which was changed to sulphur in the belief that the Latin word came from Greek. This was later interpreted as representing an f sound, resulting in the spelling sulfur. Both spelling variants (sulfur/sulphur) were common in English until the 1800s when sulphur became the standard. In other European languages the ‘f’ prevailed e.g. azufre in Spanish, schwefel in German, soufre in French and zolfo in Italian. The true Ancient Greek word for sulfur, theîon is the source of the international chemical prefix thio e.g. the amino acid methionine C5H11NO2S. In ‘English’ English ‘ph‘ remains for the ‘f‘ sound; in the late 1900s the International Union of Pure and Applied Chemistry (IUPAC) settled on (American and other ‘English’) sulfur.
**the term ‘organic’ is used here in the chemical sense, not in the environmental or ethical sense commonly used in marketing and some growing practices.
